-
PDF
- Split View
-
Views
-
Cite
Cite
Zhicheng Pan, Bangshan Wang, Ying Zhang, Yongbo Wang, Shahid Ullah, Ren Jian, Zexian Liu, Yu Xue, dbPSP: a curated database for protein phosphorylation sites in prokaryotes, Database, Volume 2015, 2015, bav031, https://doi.org/10.1093/database/bav031
Close - Share Icon Share
Abstract
As one of the most important post-translational modifications, phosphorylation is highly involved in almost all of biological processes through temporally and spatially modifying substrate proteins. Recently, phosphorylation in prokaryotes attracted much attention for its critical roles in various cellular processes such as signal transduction. Thus, an integrative data resource of the prokaryotic phosphorylation will be useful for further analysis. In this study, we presented a curated database of phosphorylation sites in prokaryotes (dbPSP, Database URL: http://dbpsp.biocuckoo.org ) for 96 prokaryotic organisms, which belong to 11 phyla in two domains including bacteria and archaea. From the scientific literature, we manually collected experimentally identified phosphorylation sites on seven types of residues, including serine, threonine, tyrosine, aspartic acid, histidine, cysteine and arginine. In total, the dbPSP database contains 7391 phosphorylation sites in 3750 prokaryotic proteins. With the dataset, the sequence preferences of the phosphorylation sites and functional annotations of the phosphoproteins were analyzed, while the results shows that there were obvious differences among the phosphorylation in bacteria, archaea and eukaryotes. All the phosphorylation sites were annotated with original references and other descriptions in the database, which could be easily accessed through user-friendly website interface including various search and browse options. Taken together, the dbPSP database provides a comprehensive data resource for further studies of protein phosphorylation in prokaryotes.
Database URL:http://dbpsp.biocuckoo.org
Introduction
As one of the most ubiquitous and important protein post-translational modifications (PTMs), the reversible protein phosphorylation was involved in almost all biological processes ( 1 , 2 ). Phosphorylation was catalysed by a protein kinase through transferring a phosphate moiety from adenosine triphosphates (ATPs) to the acceptor residue in the substrate ( 2 ). Phosphorylation in eukaryotes was extensively studied during the past decades since 1932 ( 3 ), and most of the identified phosphorylation acceptor residues were serine (Ser), threonines (Thr) and tyrosines (Tyr) ( 4 , 5 ). Protein phosphorylation had been regarded as a biological process exclusively in eukaryotes until the first evidence of the phosphorylation in bacteria, which was identified in isocitrate dehydrogenase from Escherichia coil by Garnak and Reeves ( 6 ) in 1979, while protein phosphorylation in archaea was reported in the extreme halophilic archaeon Halobacterium salinarum by Spudich and Stoeckenius ( 7 ) in 1980. Subsequently, phosphorylation in prokaryotes were extended to other residues such as histidine (His) ( 8 ), aspartic acid (Asp) ( 9 ) and cysteine (Cys) ( 10 ). It was found that His/Asp phosphorylation plays critical roles in various cellular processes such as two-component system based signaling transduction ( 11 ), while Ser/Thr/Tyr phosphorylation in prokaryotes attracted more and more attention recently ( 12 ).
Recently, rapid progresses in high-throughput (HTP) mass spectrometry based proteomic technologies greatly advanced the identification of phosphorylation sites ( 13 , 14 ). Numerous studies have been carried out to profile the phosphorylation events and advance the phosphoproteome techniques to a state-of-the-art stage ( 13 , 14 ). For example, recently Sharma et al . ( 15 ) identified over 30 000 phosphorylation events in a single human cancer cell line. Although only a handful studies have been contributed to the large-scale identification of phosphorylation in prokaryotes in comparison with eukaryotes, outstanding progresses were made by leading scientists. For example, Macek et al . ( 16 ) profiled 78 phosphorylation sites by high-accuracy mass spectrometry and biochemical enrichment of phosphopeptides from model bacterium Bacillus subtilis in 2007, and further detected 81 phosphorylation sites from the model Gram-positive bacterium Escherichia coli in 2008 ( 17 ) . Recently, 410 phosphorylation sites from 245 proteins Ming-kun were identified in Synechococcus sp. PCC 7002 by Yang et al . ( 18 ), while Reimann et al . ( 19 ) detected 801 phosphoproteins in Sulfolobus solfataricus . Besides Ser/Thr/Tyr phosphorylation, Elsholz et al . ( 20 ) profiled 121 arginine (Arg) phosphorylation sites in 87 proteins from B.subtilis in vivo . These leading studies made great contributions to expanding the understanding of molecular mechanisms and functional roles for phosphorylation in prokaryotes.
As the discoveries accumulated, the collection and maintenance of the identified phosphorylation sites became an urgent issue to be solved. Previously, a number of comprehensive databases for phosphorylation sites were constructed ( 21 ), while most of which were focused on eukaryotes. Databases such as Phosphorylation Site Database ( 22 ), SysPTM 2.0 ( 23 ), PHOSIDA ( 24 ), dbPTM 3.0 ( 25 ) and UniProt ( 26 ) have collected the prokaryotic phosphorylation sites. However, only a limited proportion of the identified prokaryotic phosphoproteins and sites were covered. In this study, we developed and presented the database of phosphorylation sites in prokaryotes (dbPSP). Totally, 7391 phosphorylation sites on seven types of phosphorylated residues including serine (Ser), threonine (Thr), tyrosine (Tyr), aspartic acid (Asp), histidine (His), cysteine (Cys) and arginine (Arg) in 3750 prokaryotic proteins from 96 organisms in 11 phyla were manually curated from the published literature. On the basis of the datasets, we analysed the sequence preferences of the phosphorylation sites and functional annotations of the phosphoproteins among eukaryotes, bacteria and archaea, while the results show that there were obvious differences among phosphorylation in the three domains of life. Taken together, the dbPSP database could serve as a comprehensive data resource for further studies of protein phosphorylation in prokaryotes.
Construction and content
The construction of database dbPSP was summarized as a diagram in Figure 1 A. We searched PubMed ( http://www.ncbi.nlm.nih.gov/pubmed ) with keywords including ‘bacteria phosphorylation’, ‘archaea phosphorylation’ and ‘archaebacteria phosphorylation’ (1 March 2014). All the retrieved 16 658 articles were manually reviewed and checked by domain experts to collect the experimentally identified prokaryotic phosphorylation sites. The curated phosphorylated residues were explicitly mapped to UniProt proteomes sequences (Release 2014_06) ( 26 ), while the annotations and cross references of phosphoproteins were also retrieved from UniProt database and integrated into the database. The references which identified phosphorylation sites were also provided in the dbPSP database.
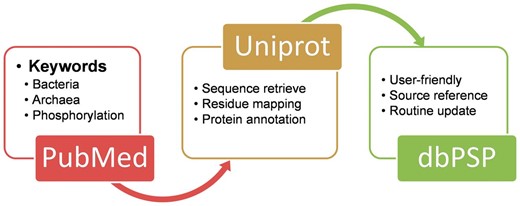
The schema of the construction processes and contents for the dbPSP database.
Besides manual curation from literatures, the prokaryotic phosphorylation sites in public databases were also collected. From databases including PHOSPHORYLATION SITE DATABASE ( 22 ), SysPTM 2.0 ( 23 ), PHOSIDA ( 24 ), dbPTM 3.0 ( 25 ) and UniProt ( 26 ), 1400, 348, 305, 186 and 176 phosphorylation sites were retrieved, respectively ( Table 1 ). These datasets were cross-checked with our manually collected dataset and integrated into dbPSP database. In total, 7391 non-redundant phosphorylation sites among seven types of residues were found in 3750 substrates from 11 phyla were provided in the database, which present a comprehensive data resource for prokaryotic phosphorylation. In total, 7171 and 209 sites were identified by HTP and low-throughput studies, respectively. Various annotations such as protein names, gene names, keywords, functional descriptions and sequence annotations from the UniProt database ( 26 ) were retrieved to annotate the collected phosphoproteins.
The comparison for the numbers of prokaryotic phosphorylation sites among dbPSP and other databases
| Database . | Sites . | Phosphoproteins . | Articles . |
|---|---|---|---|
| dbPSP | 7391 | 3750 | 174 |
| Phosphorylation Site Database | 1400 | 960 | — |
| SysPTM 2.0 | 348 | 213 | 7 |
| PHOSIDA | 305 | 282 | 4 |
| dbPTM 3.0 | 186 | 138 | 54 |
| UniProt | 176 | 135 | 73 |
| Database . | Sites . | Phosphoproteins . | Articles . |
|---|---|---|---|
| dbPSP | 7391 | 3750 | 174 |
| Phosphorylation Site Database | 1400 | 960 | — |
| SysPTM 2.0 | 348 | 213 | 7 |
| PHOSIDA | 305 | 282 | 4 |
| dbPTM 3.0 | 186 | 138 | 54 |
| UniProt | 176 | 135 | 73 |
-, Phosphorylation Site Database is not available.
The comparison for the numbers of prokaryotic phosphorylation sites among dbPSP and other databases
| Database . | Sites . | Phosphoproteins . | Articles . |
|---|---|---|---|
| dbPSP | 7391 | 3750 | 174 |
| Phosphorylation Site Database | 1400 | 960 | — |
| SysPTM 2.0 | 348 | 213 | 7 |
| PHOSIDA | 305 | 282 | 4 |
| dbPTM 3.0 | 186 | 138 | 54 |
| UniProt | 176 | 135 | 73 |
| Database . | Sites . | Phosphoproteins . | Articles . |
|---|---|---|---|
| dbPSP | 7391 | 3750 | 174 |
| Phosphorylation Site Database | 1400 | 960 | — |
| SysPTM 2.0 | 348 | 213 | 7 |
| PHOSIDA | 305 | 282 | 4 |
| dbPTM 3.0 | 186 | 138 | 54 |
| UniProt | 176 | 135 | 73 |
-, Phosphorylation Site Database is not available.
With the abundant phosphorylation sites, the distributions for different residue types and species were summarized, while the results were presented in Figure 2 . It was observed that phosphorylated serine, tyrosine, and threonine occupied 36.65%, 29.59% and 29.41% of modified residues, respectively ( Figure 2 A). The known phosphorylation sites on aspartic acid, histidine, cysteine, and arginine were limited and need further studies to explore ( Figure 2 A). In the dbPSP database, the phosphorylation sites were collected from 96 prokaryotic organisms in 11 phyla. The distribution of species at the phyla level was presented in Figure 2 B. The phylum Crenarchaeota and Proteobacteria have the most substrates with the most proportions of 39.43% and 24.10%, respectively ( Figure 2 B), while phosphorylation sites in Thermotogae and Chlamydiae/ Verrucomicrobia group were limited.
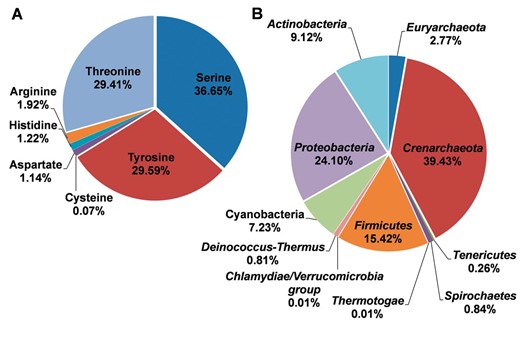
The distributions of residues types and species for the phosphoproteins in prokaryotes. ( A ) The distributions of residues types. ( B ) The distribution of phyla.
Usage
To provide convenient usage, the database was developed in a user-friendly manner, while browse and search options were provided to access the information of prokaryotic phosphorylation sites in the database. Since the phosphorylation sites are identified in different residues and various species, two browse options including ‘Browse by residue types’ ( Figure 3 A) and ‘Browse by phyla’ ( Figure 3 B) were developed in the database. Here, the serine hydroxymethyltransferase in E. coli (strain K12) was selected as an example to describe the usage of browse and search options. In the ‘Browse by residue types’, the phosphorylated residues are shown in diagrams ( Figure 3 A). By clicking the diagram of tyrosine phosphorylation, the distribution of tyrosine phosphorylated phosphoproteins in various organisms is returned ( Figure 3 A). Then the tyrosine phosphorylated phosphoproteins in Proteobacteria could be listed in a tabular format with ‘UniProt Accession’, ‘Name/Alias’ by clicking the link of ‘ Proteobacteria’ ( Figure 3 C). In the option of ‘Browse by phyla’ ( Figure 3 B), the 11 phyla in two domains including bacteria and archaea are listed for users to browse the phosphoproteins ( Figure 3 C). Through clicking on the figure of ‘ Proteobacteria’ , the distribution of phosphoproteins for different modification residue types is shown ( Figure 3 C). Then the list of tyrosine phosphorylated phosphoproteins could be retrieved after clicking the link ‘Tyrosine’, while the detailed information for specific phosphoproteins is provided by clicking protein entry ( Figure 3 D).
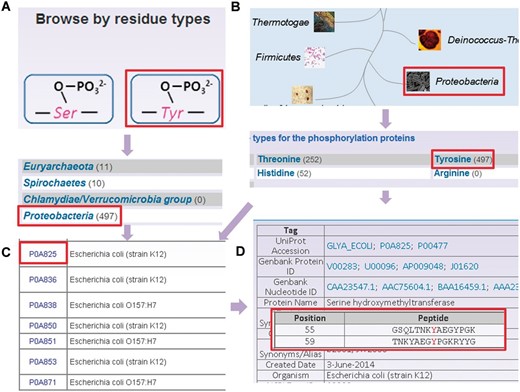
The browse options of dbPSP database. ( A ) Browse option by residue types. ( B ) Browse option by phyla. ( C ) The tyrosine phosphorylated phosphoprotein list in. ( D ) The detailed information of phosphorylated serine hydroxymethyltransferase from E. coli (strain K12).
Besides browse options, the web interface provides four search options including simple search ( Figure 4 A), ‘Advanced Search’ ( Figure 4 B), ‘Batch Search’ ( Figure 4 C) and ‘Blast Search’ ( Figure 4 D). For example, if user input the keyword ‘glyA’ in the ‘Gene Name’ area, the results will be generated in a tabular format with ‘UniProt Accession’, ‘Name/Alias’ ( Figure 4 A). Alternatively, users can use the ‘Advanced Search’ with three search terms specified in different areas and combined with three operators of ‘and’, ‘or’ and ‘exclude’, which could reduce the potential hits and provide highly related results ( Figure 4 B). Furthermore, ‘Batch Search’ is designed for retrieving multiple phosphoproteins with a list of keywords ( Figure 4 C). Finally, ‘Blast Search’ is implemented in the database to find homologous proteins with a protein sequence in Fasta Format. The NCBI BLAST package ( 27 ) is employed search related sequences ( Figure 4 D).
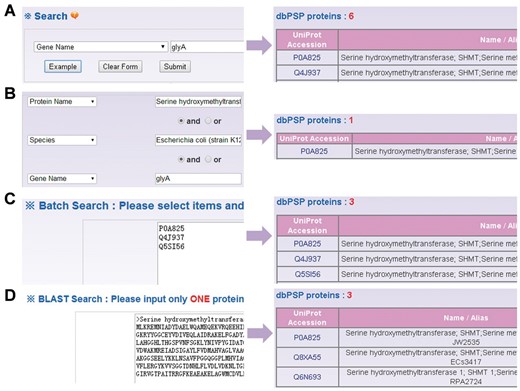
The search options of dbPSP database. ( A ) The database could be searched by simple key words. ( B ) The ‘Advanced Search’ allowed users to submit up to three terms for search. (C) The ‘Batch Search’ for retrieving multiple protein entries with a list of terms. ( D ) The database could be queried with a protein sequence to find identical or homologous phosphoproteins.
Discussion
As one of most important protein PTMs, prokaryotic protein phosphorylation was critical for numerous cellular processes through modification of various types of residues ( 28 , 29 ). After the first discovery of phosphorylation events in prokaryotes, a large number of substrates and sites have been identified to dissect the molecular mechanisms and functional roles of phosphorylation. Although previously various databases were developed to maintain the known phosphorylation sites, most of these databases were focused on eukaryotes. In this regard, an integrated and comprehensive database for prokaryotic phosphorylation is urgently needed. In this study, we presented a manually curated and comprehensive database of dbPSP, which aimed to maintain known phosphorylation sites from various organisms in prokaryotes.
Previously, numerous studies on eukaryotes indicated that phosphorylation was mediated by linear motifs ( 5 , 30 ). With the dataset collected in this study, we analysed the sequence preferences and motifs for Ser/Thr phosphorylation in bacteria ( Figure 5 A), archaea ( Figure 5 B) and eukaryotes ( Figure 5 C), while 10 092 eukaryotic Ser/Thr phosphorylation sites from phospho.ELM database were employed for comparison ( 31 ). As the sequence preferences illustrated by WebLogo ( 32 ), alanine and lysine has high frequencies around the phosphorylation sites in bacteria ( Figure 5 A) and archaea ( Figure 5 B), respectively, there were abundant serine and glutamic acid around the phosphorylated residues ( Figure 5 C). To further dissect the differences, pLogo was employed to pairwisely compare the sequence preferences ( Figure 5 D–F) ( 33 ). It was observed that positively charged residues including arginine and lysine were enriched around phosphorylated Ser/Thr in archaea than bacteria ( Figure 5 D) and eukaryotes ( Figure 5 F), while proline were over-presented in +1 position of the phosphorylation sites in eukaryotes than bacteria ( Figure 5 E) and archaea ( Figure 5 F). Taken together, obvious differences were observed among the sequence preferences of phosphorylation sites in the three domains of organisms.
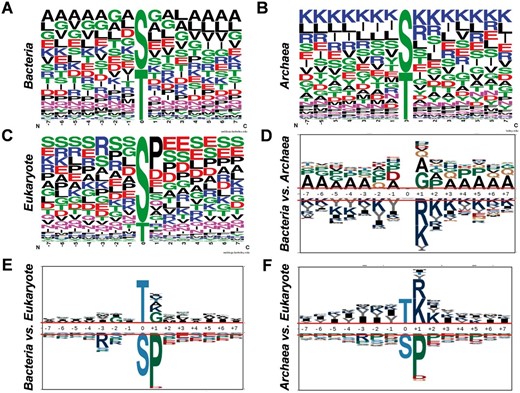
Analyses of sequence preferences of phosphorylation sites in prokaryotes. The sequence preferences of phosphorylation sites in bacteria ( A ), archaea ( B ) and eukaryotes ( C ) were presented with WebLogo. The comparisons of sequence preferences for bacteria and archaea ( D ), bacteria and eukaryotes ( E ), archaea and eukaryotes ( F ).
Furthermore, with the comprehensive phosphorylation datasets in the dbPSP database, we tried to analyze the functional annotations of phosphoproteins in prokaryotes with the examples of E.coli (strain K12) ( E. Coli k12 ) and Sulfolobus acidocaldarius , which contained the most identified phosphoproteins and sites in bacteria and archaea, respectively. The gene ontology (GO) (31 March 2012) association files were downloaded from the The Gene Ontology Annotation (GOA) database at the European Bioinformatics Institute (EBI) ( http://www.ebi.ac.uk/goa ) ( 34 ) and the complete proteomes were retrieved from AmiPro Database ( 26 ). With hypergeometric distribution ( 35 ), we statistically analysed the enriched biological processes, molecular functions and cellular components for phosphoproteins in E. Coli k12 ( Figure 6 A, P- value < 10 −9 ) and S. acidocaldarius ( Figure 6 B, P- value < 10 −2 ). It was observed that translation (GO:0006412) was the intensively enriched biological process in phosphoproteins from E. Coli k12 ( Figure 6 A), while translation-related annotations of tRNA aminoacylation for protein translation (GO:0006418) and regulation of translational fidelity (GO:0006450) were also over-presented in phosphoproteins from S. acidocaldarius ( Figure 6 ). For molecular functions, phosphoproteins from E. Coli k12 and S. acidocaldarius both enriched annotations of nucleotide binding (GO:0000166) ( Figure 6 ). Furthermore, phosphoproteins from E. Coli k12 over-presented other molecular functions including structural constituent of ribosome (GO:0003735), rRNA binding (GO:0019843), protein binding (GO:0005515), magnesium ion binding (GO:0000287), identical protein binding (GO:0042802) and RNA binding (GO:0003723) ( Figure 6 A), while phosphoproteins from S. acidocaldarius enriched aminoacyl-tRNA ligase activity (GO:0004812), ligase activity (GO:0016874), aminoacyl-tRNA editing activity (GO:0002161), nucleic acid binding (GO:0003676) and ATP binding (GO:0005524) ( Figure 6 B). In addition, a handful of cellular components were over-presented in phosphoproteins from E. Coli k12 ( Figure 6 A), while no enrichment was observed in for S. acidocaldarius .
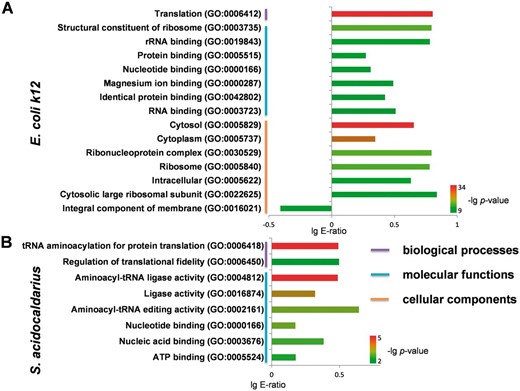
Statistical analyses of GO annotation for phosphoproteins in E. Coli k12 and S. acidocaldarius. ( A ) The enriched GO terms for phosphoproteins in E. Coli k12 . ( B ) The enriched GO terms for phosphoproteins in S. acidocaldarius .
Taken together, in this study the dbPSP database was developed to maintain the experimentally identified phosphorylation sites in prokaryotes. We anticipated that such database could provide a useful resource for further studies and understanding of phosphorylation in prokaryotes.
Funding
This work was supported by grants from the National Basic Research Program (973 project) (2013CB933900 and 2012CB910101); Natural Science Foundation of China (31171263, 81272578 and J1103514); International Science and Technology Cooperation Program of China (2014DFB30020); and China Postdoctoral Science Foundation (2014M550392). Funding for open access charge: 31171263.
Conflict of interest . None declared.
References
Author notes
† These authors contributed equally to this work.
Citation details: Pan,Z., Wang,B., Zhang,Y., et al . dbPSP: a curated database for protein phosphorylation sites in prokaryotes. Database (2015) Vol. 2015: article ID bav031; doi:10.1093/database/bav031



